Page 47 - Memora anual Fundación CIEN 2015
P. 47
3. SCIENTIFIC ACTIVITY
and clinical impairment". Reference protocol: • “NAC” Phase III study: "Effect of Adjuvant
CLOZAPINE-1, Nº EudraCT: 2006-00200-34. PI: Dr. treatment with N-acetylcysteine for 48 weeks
Francisco Javier Sanz Fuentenebro. 2010-2013. over the loss of gray matter and oxidative
CIBERSAM. metabolism in patients with early onset
psychotic episodes". Randomized, Double-blind,
• “ABE_4869g” A phase II randomized, double- placebo-controlled Clinical Trial Code: FIBHGM-
blind, placebo-controlled, parallel group, ECNC002-2012, EudraCT Number:
multicenter to evaluate the efficacy and safety 2012-005435-87. Promoter Center: Foundation
of MABT5102A in patients with moderate for Biomedical Research Gregorio Maranon
Alzheimer's disease". Code EudraCT: (2010- Hospital. PI: Celso Arango.
021926-37). GENENTECH, Inc.
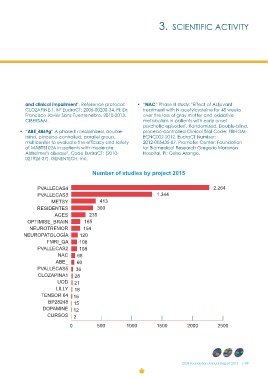
Number of studies by project 2015
PVALLECAS4 2.264
PVALLECAS3
1.344
METSY
RESIDENTES 413
360
AGES 235
OPTIMISE_BRAIN 165
NEUROTREMOR 154
NEUROPATOLOGÍA 120
108
FMRI_QA 108
PVALLECAS2 68
60
NAC 36
ABE_ 28
PVALLECAS5 21
CLOZAPINA1 18
UOD 16
LILLY 15
TENSOR 64 12
BP28248 2
DOPAMINE
CURSOS
0 500 1000 1500 2000 2500
CIEN Foundation Annual Report 2015 / 49